V.Ryan © 2020
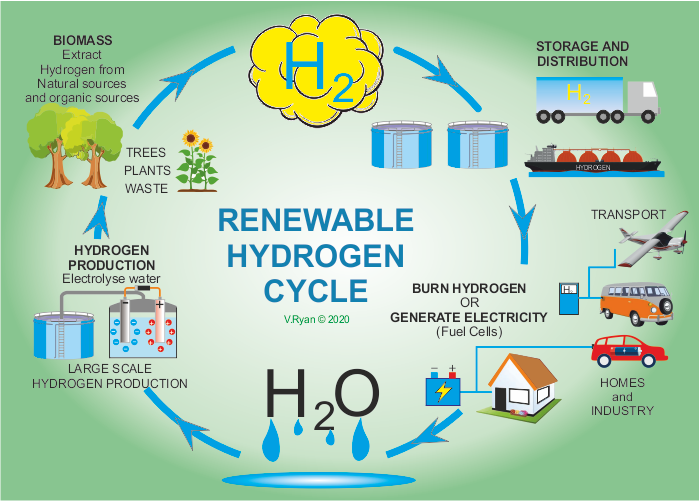
The hydrogen cycle is the conversion of water to hydrogen and oxygen, followed by its transformation to fuel and electricity and finally its ‘recycling’ back to water.
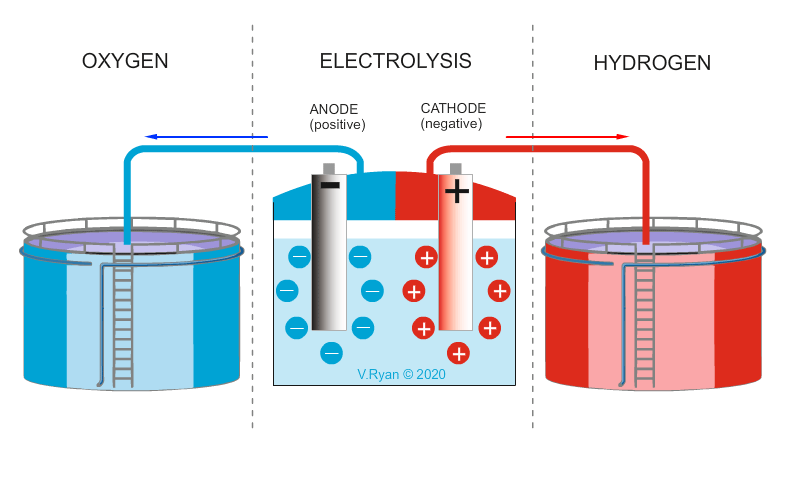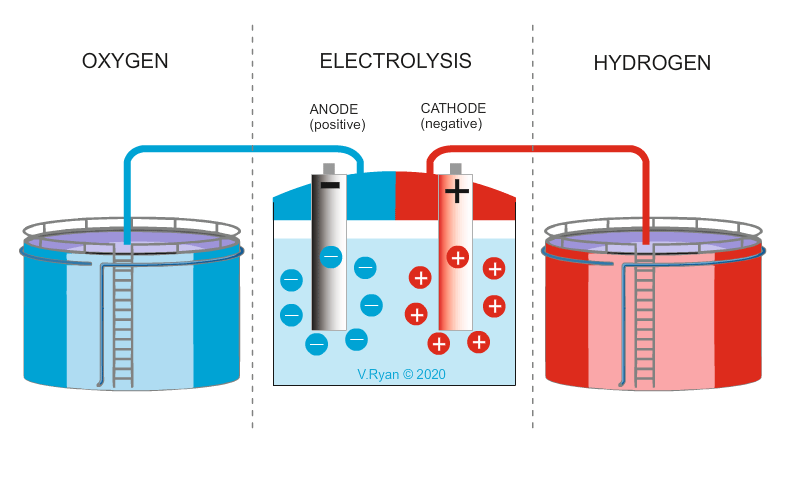
The hydrogen cycle starts with water (H2O). Hydrogen and oxygen is split through a process called electrolysis. Hydrogen can also be extracted from natural materials such as wood, plants and other organic materials. The extracted hydrogen can be burnt by conventional engines or preferably, used in hydrogen cells, to produce electricity. The only waste / exhaust produced by hydrogen cells is pure water.
The main problem with hydrogen production, is access to a clean energy source (wind, solar, nuclear, wave, tidal, hydro), to produce the electricity required to split water into hydrogen and oxygen. If power stations fuelled by coal and oil, are used to provide the power, pollution is the result. Further to this, the storage of hydrogen is costly. Also, the purer the water, the easier it is, to split into its two elements. Less than pure water, incurs additional costs.
2. Hydrogen fuel cells are efficient, operating at an efficiency of 80%. This compares very well with electricity generated at a power station and transmitted over power lines. The generation of electricity by burning fossil fuels at a power station, is only 35% efficient. Furthermore, 10% of the electricity transmitted over power lines is lost, due to electrical resistance.
3. The emissions from hydrogen fuel cells, are in the form of water / water vapour and are non-polluting.
4. Fuel cells have a wide range of applications. They can be used to ‘power’ vehicles, houses and businesses.
5. Comparisons between cars powered by fuel cells and those by petrol, show that fuel cells are much more energy efficient.
6. Moving to an hydrogen economy will reduce pollution, environmental damage and global warming.
2. Fuels cells are most efficient within a specific temperature range. Operating outside the this range, leads to a drop in efficiency.
3. In order to establish an hydrogen economy, there is a need to invest heavily in infrastructure, so that hydrogen can be moved as a gas and liquid.
4. One aim of the hydrogen economy is to dramatically reduce pollution. This can only be achieved if alternative energy is used to produce the electricity required for electrolysis. If electricity is produced by fossil fuels, pollution and environmental damage continues.
5. Hydrogen fuel cells are currently expensive, but costs are falling. As demands for this technology grows, the increase in scale of production, should reduce costs.