| CLICK HERE FOR INDEX PAGE | |
| PLASTICS - QUESTIONS AND ANSWERS | |
| V. Ryan © 2010 | |
| PDF FILE - CLICK HERE FOR PRINTABLE WORKSHEET | |
|
|
|
| It is recommended that you read and understand the information on Plastics, included earlier in this section of the website. | |
| THERMOSET POLYMERS | |
| 1. Explain the term ‘thermoset polymer’, with reference to molecular structure. | |
| Once 'set' these plastics cannot be reheated to soften, shape and mould. The molecules of these plastics are cross linked in three dimensions and this is why they cannot be reshaped or recycled. The bond between the molecules is very strong. | |
| 2. Thermoset polymers are very useful in the manufacture of electrical fittings. Name a thermoset polymer used for this purpose | |
| Urea Formaldehyde (UF) | |
| 3. Describe the properties of the polymer you have named above that make it suitable for electrical fittings. | |
| Urea Formaldehyde has physical properties of high hardness and high toughness, making it suitable for strong, knock-resistant electrical fittings. It is also scratch resistant and a very good electrical insulator, making electrical fittings manufactured from this polymer safe to use. | |
| THERMOPLASTICS | |
| 4. Explain the term ‘thermoplastic’, with reference to molecular structure. | |
| These plastics can be re-heated and therefore shaped in various ways due being long chain monomers that are not inter- connected. They become mouldable after reheating as they do not undergo significant chemical change. Reheating and shaping can be repeated. The bond between the molecules is weak and become weaker when reheated, allowing reshaping. These types of plastics can be recycled. | |
| 5. Thermoplastics are very useful in the manufacture of mobile phone casings. Name a thermoplastic used for this purpose. | |
| Polycarbonate | |
| 6. Describe the properties of the polymer you have named above that make it suitable for mobile phone casings. | |
| Polycarbonate is a thermoplastic which means it can be shaped and formed through a number of manufacturing processes. It machines well and can be solvent bonded and welded. It is tough and resistant to damage which is an ideal property for a mobile phone. If dropped, a mobile phone with a polycarbonate casing is likely to survive undamaged. It is an insulator, often used to insulate electrical circuits. It is supplied in a range of colours. | |
| FURTHER QUESTIONS: | |
| 7. List two more thermosetting plastics and describe practical applications of each one. | |
| NAME 1: Polyester Resins. | |
| PRACTICAL APPLICATION: If resins are combined with a material such as fibre glass the result is a very tough material that can resist impact. This type of material is known as a glass reinforced plastic (GRP) and is used in car body repairs, sailing boats, corrugated sheet because of its lightness, toughness and resistance to water. | |
| NAME 2: Melamine Formaldehyde. | |
| PRACTICAL APPLICATION: Used in the production of plastic laminates because of its smooth surface and hygienic qualities. It is also used in electrical plugs and sockets because it can be cast and it is an excellent insulator. | |
| 8. List two more thermoplastics and describe practical applications of each one. | |
| NAME 1: Polythene. | |
| PRACTICAL APPLICATION: Can be moulded into almost any form due to its excellent moulding qualities. Used for the production of bottles, bowls, toys, tube etc... It is available in large sheets. There are two types: High density which is rigid and hard, and low density which is tough and flexible. Machine parts are generally made from high density polystyrene whilst bottles are made from the low density polystyrene. | |
| NAME 2: Polyvinyl Chloride. | |
| PRACTICAL APPLICATION: Better known as PVC. It is a tough material which can be purchased as a hard material or alternatively a flexible form. It can be welded or bonded with an adhesive. It has a range of uses including water pipes, raincoats, long play records, coating on electrical wires and many more. | |
| 9. Explain the term MONOMER, with the aid of a diagram(s) and notes. | |
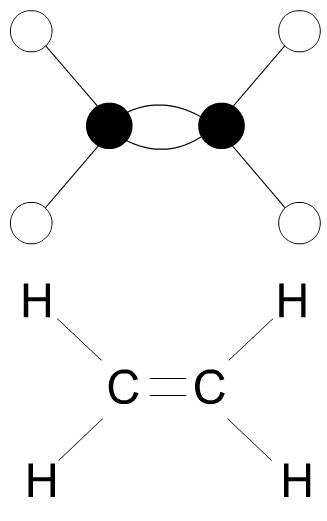 |
A MONOMER is a small
molecule that combines chemically to other monomers to form a polymer Example - from oil - the hydrocarbon ethylene (seen opposite). Many modern plastics are manufactured from oil. |
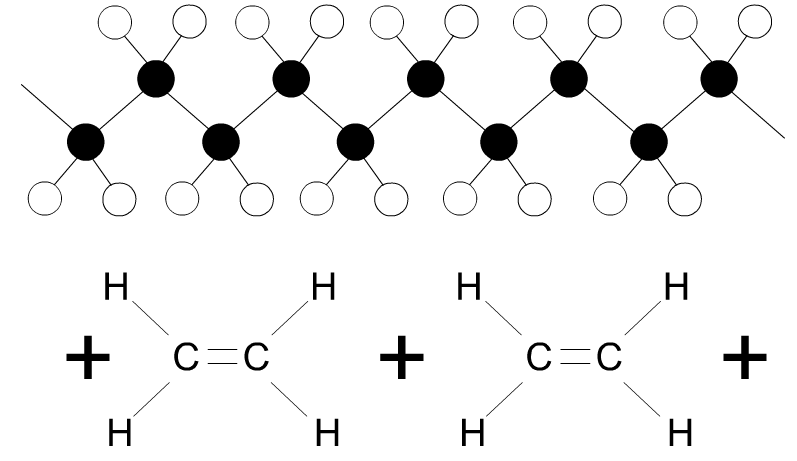
| 10. Explain the term LONG CHAIN MONOMER, with the aid of a diagram(s) and notes. Include the name of a thermoplastic, composed of long chain monomers. | |
| An ethylene monomer forms a’ long chain monomer’ due to individual ethylene monomers joining together. This produces the polymer polyethylene | |
 |
|
| CLICK HERE FOR RESISTANT MATERIALS INDEX PAGE | |