| |
| CLICK HERE FOR INDEX PAGE |
| |
| GALVANISING STEEL AND IRON - 1 |
| V. Ryan © 2008 - 2016 |
| |
| VIDEO - GALVANISING IRON AND STEEL |
 |
| |
| |
PDF FILE - CLICK HERE FOR PRINTABLE WORKSHEET
POWERPOINT FILE - CLICK HERE FOR PRINTABLE WORKSHEET
Steel is a very strong and versatile metal used to
manufacture a large range of products. Steel surrounds us in our every day
lives as it is used to construct buildings and structures. It is a
versatile material although it has one weakness, corrosion.
If an exposed steel surface comes in contact with water or moisture rust
can take hold. Rust can damage the surface of the steel as seen on
corroded car bodywork. It is possible that on a large structure such as a
bridge rust can cause structural failure leading to collapse. |
| |
| Steel is usually coated. For example, the Golden Gate
Bridge in San Francisco receives a new coat of rust resistant bronze paint
every five years. This prevents rust damaging the structural integrity of
the bridge. On smaller manufactured items zinc is used to coat steel and
this is an effective measure against rust. This process is called
galvanising. |
| |
|
|
| |
| The bolt seen opposite shows the damage caused by rust.
If this occurs to the bolts holding together the parts of a bridge, the
bridge would be in danger of collapsing. |
|
 |
| |
|
|
| The steel tie ring (below) has also been attacked by
rust. The magnified surface shows how deep the damage is and this will
eventually mean that it needs replacing. |
|
 |
| |
|
|
| The galvanised tie ring is almost the same design.
However, there is one crucial difference - it has been galvanised. The
magnified surface shows the layer of zinc that protects the inner steel
from the elements. This tie ring will last for many years whist the
original unprotected steel tie ring (above) will last only two or three
years. |
|
 |
| |
|
|
1. Research the internet
and compare the price of galvanised nuts and bolts to the steel
equivalent.
2. List a range of steel products that are
also supplied as galvanised.
3. Research how steel is galvanised. |
| |
|
|
| |
| HOT DIPPING - GALVANISING |
| |
Galvanised steel is steel that has been coated with
zinc in order to prevent rusting / corrosion. Sometimes the galvanising
process is referred to as hot dip galvanising. The zinc forms a barrier
against corrosion in that the steel underneath does not come into contact
with water / moisture in the air.
The hot dipping process applies quite a think layer of zinc to the steel
by passing the steel through a molten bath of zinc. The temperature of the
zinc is usually in the region of 460 degrees centigrade. The zinc forms a
bond with the steel by forming an iron-zinc alloy. The zinc also forms a
zinc oxide when it comes in contact with the air which also helps prevent
corrosion. |
| |
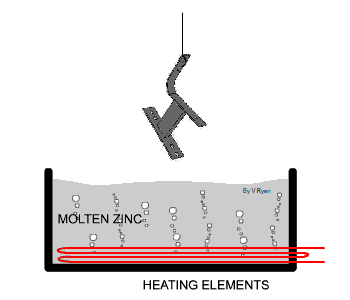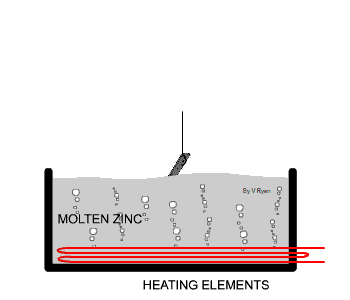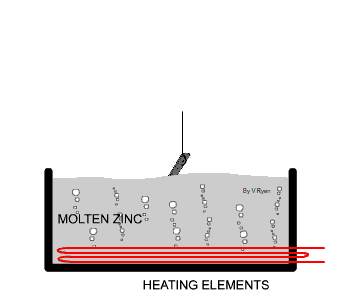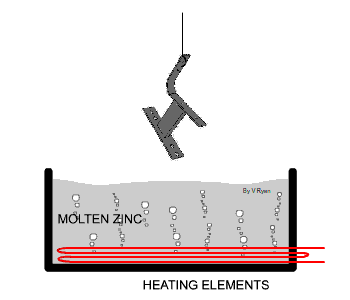
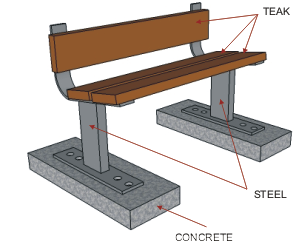
| The steel used in the manufacture of the bench seen
below has been protected by adding a zinc coating. This is done by
submerging the steel parts in molten zinc. The zinc becomes part of the
steel outer layer. |
| |
 |
|
 |
| |
|
|
| |
| CLICK HERE FOR SECOND PAGE ON GALVANISING STEEL |
| |
| CLICK HERE FOR EQUIPMENT AND PROCESSES
INDEX PAGE |
| |
| CLICK HERE FOR RESISTANT MATERIALS INDEX
PAGE |
| |





