| |
| CLICK HERE FOR INDEX PAGE |
| |
| GALVANISING STEEL AND IRON - 2 |
| V. Ryan © 2008 - 2021 |
| |
| VIDEO - GALVANISING IRON AND STEEL |
| |
 |
| |
| PDF FILE - CLICK HERE FOR
PRINTABLE WORKSHEET |
| |
| POWERPOINT FILE - CLICK HERE FOR PRINTABLE WORKSHEET |
| |
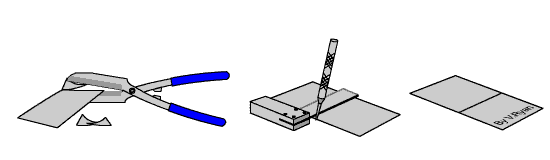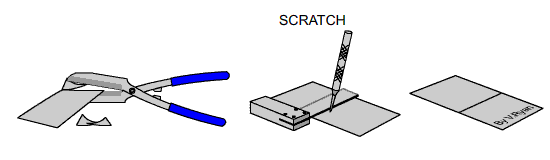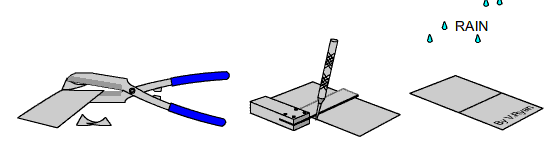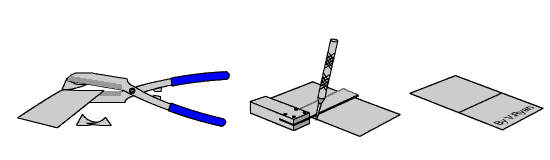
TASK
Carefully cut a small piece of galvanised steel sheet with tin snips. The
edges will be shape so handle it with care. Scratch the surface with an
engineers scriber and leave it outside in the rain for a few days. Compare
it to a piece that has not been scratch and has also been left outside for
the same length of time. |
| |
 |
| |
|
|
| |
| What do you think will happened
to the surface of each piece of galvanised steel? |
| |
 |
|
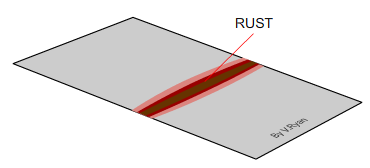
You will probably find that rust has formed along the
scratched line. If the steel was to be left outside for longer the rust
would spread out and damage the surface considerably. Eventually the rust
would ‘eat’ its way through the thin sheet metal. It would eventually be
possible to bend and break the steel quite easily. |
| |
|
|
| |
| ELECTROPLATING - ELECTRO-GALVANISED |
| |
| In industry electroplating is often the process used to
galvanise steel products as it is more efficient and more cost effective
than the hot dipping process. Also, it is easier to control the entire
process. For instance, the thickness of the coating can be determined with
great accuracy. |
| |
A good example of this can be seen in car
manufacturing. Galvanising of steel car bodies is now standard practise
although twenty years ago only the must prestigious of cars such as Rolls
Royce had galvanised bodies.
The steel car body is electrically charged as negative and is suspended in
a conducting solution known as the electrolyte. As the car body is
negatively charged it is known as the cathode. Rods of pure zinc are
positively charged and it is the zinc from these rods that is eventually
deposited on the surface of the steel. The rods are suspended in the
electrolyte.
A current passing through the electrolyte causes the zinc already in the
solution to migrate towards the cathode (the car body). At the same time
zinc from the anode (the zinc rods) pass into the electrolytic solution.
The rods eventually need replacing. |
| |
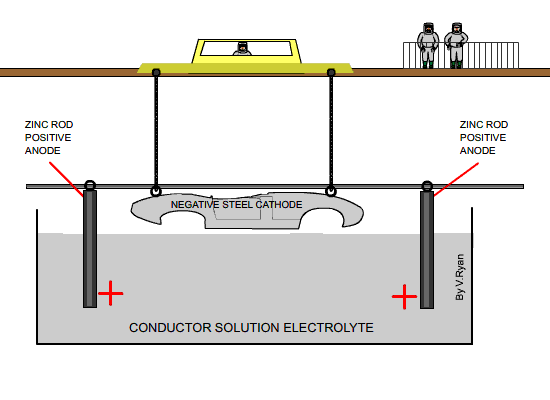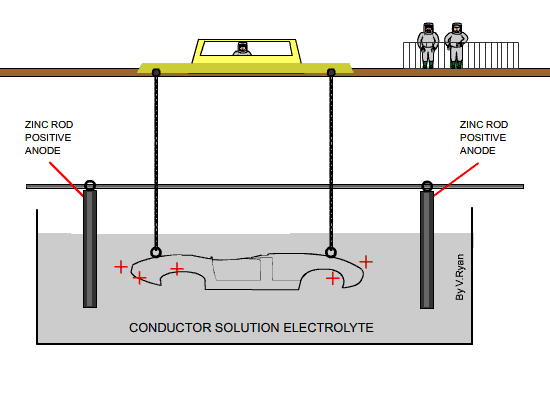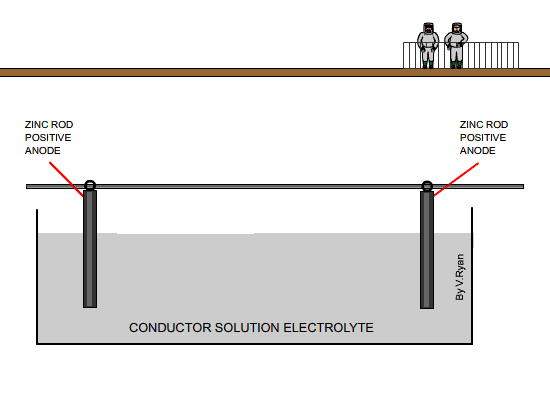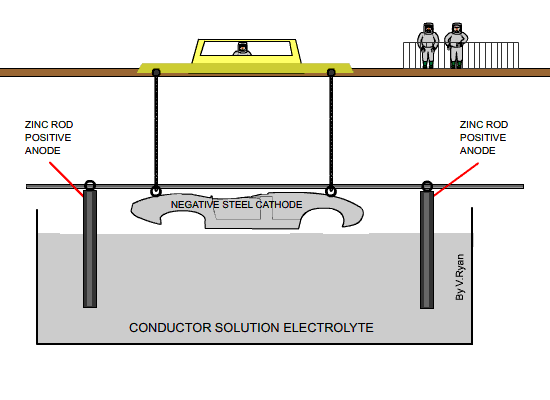 |
| The diagram above shows a car body about to be
lowered into the electrolyte solution |
| |
| 1. List a range of products that have been treated in an electrolyte solution and galvanised. |
| |
| |
| |
| |
| 2. Why are car bodies galvanised? |
| |
| |
| |
| |
|
|
| |
| |
| CLICK HERE FOR FIRST PAGE ON GALVANISING STEEL |
| |
| CLICK HERE FOR EQUIPMENT AND PROCESSES
INDEX PAGE |
| |
| CLICK HERE FOR RESISTANT MATERIALS INDEX
PAGE |
| |



